Purpose
The predominant pattern of relapse for patients with B-cell non-Hodgkin lymphoma (NHL) who undergo anti-CD19 Chimeric Antigen Receptor T-cell Therapy (CART) involves sites previously involved by NHL. Bridging radiotherapy (BRT) has shown to be an effective bridging treatment with excellent local control rates. Our previous work defined limited/oligometastatic lymphoma as disease involving <5 sites. This study is the first to report on the role of BRT for patients with limited (<5 involved sites) disease prior to CART.
Methods
Following Institutional Review Board approval, a retrospective review from 2018 to 2023 across 3 Mayo Clinic sites (Arizona, Florida, and Minnesota) was performed and identified 139 patients with relapsed/refractory (r/r) B-cell NHL without bone marrow involvement who received CART and had <5 disease sites prior to leukapheresis. Ann Arbor staging was used to define the number of involved disease sites. An involved disease site included the Ann Arbor anatomical lymph node region(s) harboring the pathological lymph node(s) or any extranodal lesion. Bridging treatment (radiation and/or systemic therapy) was defined as treatment administered between leukapheresis and CART infusion. The primary endpoint was event-free survival (EFS),defined from CART infusion to any disease progression or initiation of post-CART consolidative/salvage therapy. Secondary endpoints included progression -free survival (PFS),defined from CART infusion to any disease progression, and overall survival (OS), defined from CART infusion to death.
Results
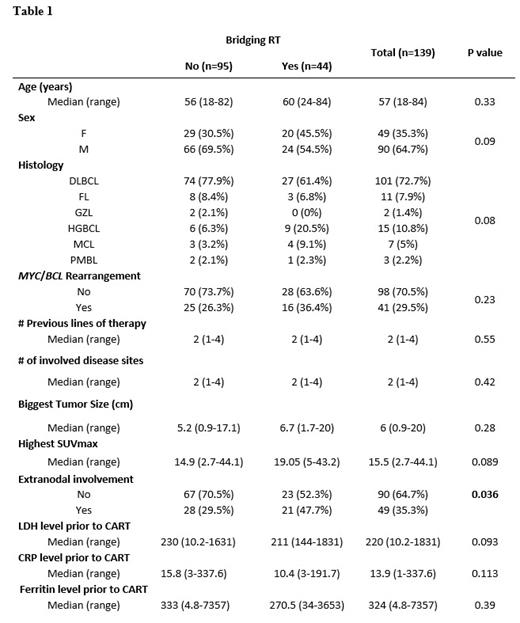
Prior to CART infusion, 44 (32%) patients received BRT and 95 (68%) did not. Bridging systemic therapy was given to 5 (11%) and 34 (36%) patients in the BRT and no BRT groups, respectively (p=0.003). The median number of involved disease sites prior to leukapheresis was 2 (range: 1-4). There was no significant difference in the baseline characteristics prior to CART infusion between the BRT and no BRT groups, except for extranodal involvement that was more common in the BRT group (Table 1). The median equivalent 2 Gy dose was 27 Gy (Interquartile range 23-34), and the most common BRT regimen was 20 Gy in 5 fractions. Among the 44 BRT patients, 34 received radiation to all visible PET-avid disease sites, and 10 received radiation to some but not all disease sites.
The median follow-up was 20 months. The rate of grade ≥2 cytokine release syndrome was 41% in both groups (p=0.99). The rate of grade ≥2 immune effector cell-associated neurotoxicity syndrome was 21% and 37% in the BRT and no BRT groups, respectively (p=0.059). There were no observed grade ≥3 BRT-related toxicities. Objective response rate by PET 5 point scale was higher in the BRT group (93% versus 79%; p=0.036). Crude rates of progression were 30% in BRT and 55% in no BRT patients, with 9/13 (69%) and 43/52 (83%) patients relapsing in pre-existing sites of disease present prior to CART infusion in the BRT and no BRT group, respectively. Seven (16%) BRT patients relapsed in the radiation field. The 2-year EFS was 50% in the BRT group and 39% in the no BRT group (p=0.01). The 2-year PFS was 55% in the BRT group and 45% in the no BRT group (p=0.017). The 2-year OS was 55% in the BRT group and 62% in the no BRT group (p=0.7). Intriguingly, patients who received BRT to all visible PET-avid disease sites (n=34) had further improvement in 2-year EFS (67% vs 39%, p=0.003) and 2-year PFS (71% vs 46%, p=0.008) compared to no BRT or patients who received BRT to some but not all visible disease sites (n=105).
Nine patients (2 in BRT and 7 in no BRT groups) received consolidative radiotherapy for day 30 residual disease with only one patient subsequently experiencing progression. Twenty-five patients (6 in BRT and 19 in no BRT groups) received salvage/palliative RT for post-CART relapsed disease. Thirty-seven (39%) patients in the no BRT group remained without disease progression and did not receive radiation in the bridging, consolidative or salvage/palliative setting.
Conclusion
Bridging radiotherapy prior to CART infusion for patients with limited (<5 involved disease sites) r/r NHL appears to improve response rate, EFS, and PFS without causing significant toxicities. Approximately one third of patients who do not receive BRT will require subsequent post-CART radiation, and another third will remain free of radiation and disease progression.
Disclosures
Ansell:Affirmed: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research; ADC Therapeutics: Other: Contracted Research. Paludo:Biofourmis: Research Funding; Karyopharm: Research Funding; AbbVie: Consultancy. Villasboas:Regeneron: Research Funding; Aptose: Research Funding; Epizyme: Research Funding; Enterome: Research Funding; CRISPR: Research Funding; Genentech: Research Funding. Wang:Morphosys: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Genentech: Research Funding; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Alhaj Moustafa:Abbvie: Consultancy; CSL Behring: Consultancy; Acrotech Biopharma: Research Funding. Murthy:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Senti Biosciences: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bavarian Nordic: Membership on an entity's Board of Directors or advisory committees. Hoppe:Merck SAC for AHOD1822/KN667 low risk: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal